Background
Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia characterized by chronic hemolysis mediated by classical complement pathway activation. Chronic inflammation and anemia associated with CAD can lead to profound fatigue and general weakness, impacting the patients' quality of life. Sutimlimab is a first-in-class humanized monoclonal antibody approved for the treatment of CAD. To assess the effect of sutimlimab on patients' self-reported fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-Fatigue] scores) in CAD, a post-hoc analysis of the CADENZA Part A study was carried out.
Objective
To assess the effect of sutimlimab vs placebo on FACIT-Fatigue total score, and individual items of the FACIT-fatigue scale in patients with CAD.
Methods
CADENZA (NCT03347422) Part A was a phase 3, double-blind, 26-Week study where patients were randomized (1:1) to receive intravenous sutimlimab or placebo.
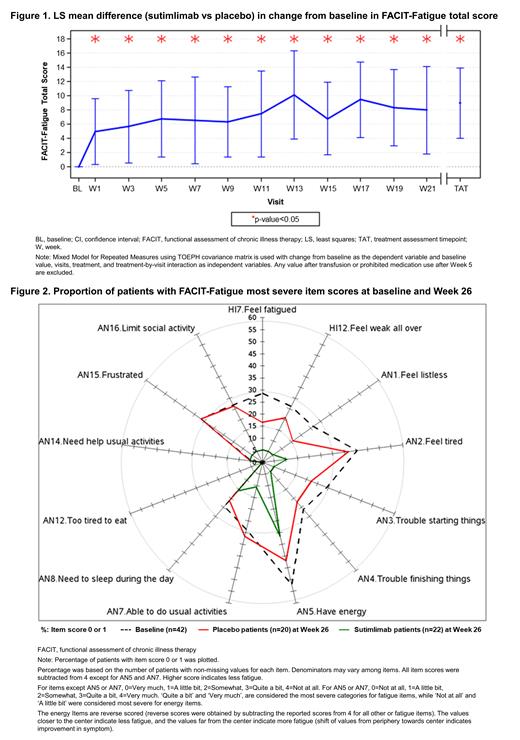
The 13-item FACIT-Fatigue questionnaire comprising 11 fatigue items and 2 energy items (‘I have energy’ or 'I am able to do my usual activities') was scored on a 0 (“not at all”) to 4 (“very much”) response scale, and the total scores ranged from 0 to 52; higher scores indicated less fatigue. Least square (LS) mean difference (sutimlimab vs placebo) in change from the baseline (BL) in FACIT-Fatigue total score were estimated at all visits using mixed model for repeated measures. Treatment assessment timepoint (TAT) was the average of values from Week 23, 25, and 26 visits. Patients were considered responders if they achieved a change from BL in FACIT-Fatigue total score of ≥5, did not receive blood transfusions from Week 5 through TAT, and did not take any protocol prohibited CAD medications. Patients with missing data at a given visit were considered non-responders. Time to first response to treatment was assessed using the Kaplan-Meier product limit method. For the item level analysis of the FACIT-Fatigue scale, the proportion of patients reporting the two worst response options 'Quite a bit' or ‘Very much’ for fatigue items and ‘Not at all’ or ‘A little bit’ for energy items was calculated at BL and Week 26.
Results
A total of 42 patients were included in the study (sutimlimab group: n=22; placebo group: n=20). The median (range) age of patients was 66.0 (46-88) years and 78.6% (n=33) were female. The mean (SD) FACIT-Fatigue total score for the pooled population at BL was 32.30 (11.83). About 79% of patients (n=33) had a total score below the general healthy population mean of 43. The LS mean (95% CI) difference (sutimlimab vs placebo) in change from BL in the FACIT-Fatigue total score was 4.97 (0.35─9.60) at Week 1 and 8.93 (4.00─13.85) at TAT. The nominal significant difference that started at Week 1 (rapid treatment effect) was sustained throughout Part A ( Figure 1). Overall, the fatigue response rate was higher in the sutimlimab group than placebo group throughout the study. The median time (95% CI) to first FACIT-Fatigue response in the sutimlimab and placebo groups were 3.1 (1.1─3.1) and 9.1 (3.3─22.4) weeks (log-rank test p<0.05), respectively. At BL, ~25-30% of the patients had reported the two worst response options (‘Quite a bit’ or 'Very much') for most of the FACIT-fatigue items. Around 40.5% (17/42) of the patients responded with one of the two worst options for item 'I feel tired', and more than half of the patients (22/42; 52.4%) reported one of the two worst options (‘Not at all’ or 'A little bit') for item 'I have energy'. At Week 26, lower proportion of patients in sutimlimab group reported one of the two worst options for fatigue and energy items compared with the placebo group ( Figure 2).
Conclusion
Sutimlimab treatment led to rapid and sustainable improvement in FACIT-Fatigue scores and higher response rates as compared to placebo. Number needed to treat to observe a sutimlimab-related FACIT-Fatigue benefit was 4. The proportion of severe FACIT-Fatigue answers for fatigue and energy items was noticeably lower in sutimlimab-treated patients at Week 26 compared to placebo, highlighting the effect of sutimlimab in improving almost all dimensions of FACIT-Fatigue scale.
Disclosures
Roeth:Biocryst: Consultancy, Honoraria; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bioverativ: Consultancy, Honoraria; Apellis Apellis Pharmaceuticals: Consultancy, Honoraria. Ueda:Chugai: Consultancy, Honoraria, Research Funding; Asahi Kase: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SOBI: Consultancy, Honoraria. Bozzi:Sanofi: Current Employment, Current equity holder in publicly-traded company. Patel:Sanofi: Current Employment. Karaouni:Sanofi: Consultancy. Yoo:Sanofi: Current Employment, Current equity holder in publicly-traded company. Cella:Bristol Myers Squibb: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Celcuity: Consultancy; Fulcrum: Consultancy, Research Funding; Ipsen: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Bayer: Research Funding; Clovis: Research Funding; Ionis: Research Funding. Wang:Sanofi: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal